-
iestinstrument
Unveiling Electrochemistry: A Guide to In-Situ EIS Testing
1. Preface
With the widespread application of lithium-ion batteries in consumer electronics, electric vehicles, and energy storage systems, the requirements for fast-charging performance have become increasingly stringent. Electrochemical impedance spectroscopy (EIS) is a critical and widely employed technique for evaluating the dynamic performance of these batteries. For a detailed description of the measurement procedures, please refer to the article “Exploring Electrochemistry | Overview of Lithium-ion Battery Electrochemical Impedance Spectroscopy” on IEST’s official public account. In the research of lithium battery materials and processing performance, engineers may also need to evaluate the EIS characteristics of battery cells under various conditions—such as during charge–discharge processes at different states of charge (SOC)—which falls under the category of in-situ EIS testing.
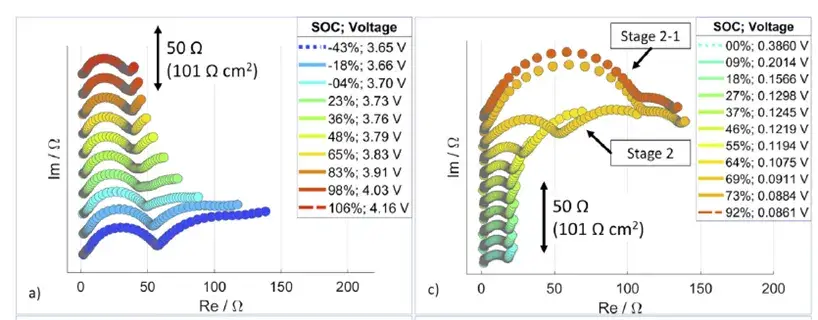
As shown in Figure 1, Pouyan Shafiei Sabet (first author and corresponding author) and Dirk Uwe Sauer from RWTH Aachen University, Germany, conducted an in-depth analysis of the EIS spectra of high energy density lithium-ion batteries (NCM/graphite system). Their work elucidated that the reaction processes depicted in the full-cell impedance spectra correspond to the electrochemical reactions occurring at both the positive and negative electrodes. This finding is of significant importance for understanding the reaction mechanisms in lithium-ion batteries. This article provides a preliminary introduction to the testing procedure and application scenarios of in-situ EIS.
Figure 1. EIS curves of an NCM half-cell at different SOCs
2. Experimental Equipment and Testing Methods
2.1 Experimental Equipment:
The testing was conducted using the ERT7008-100mA model (IEST), as shown in Figure 2.
Figure 2. Appearance of the ERT7008
1.2 Testing Method:
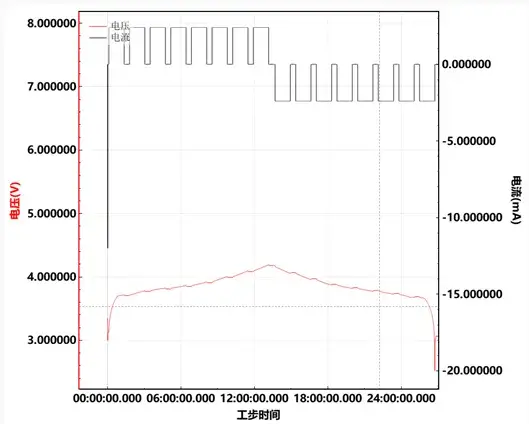
A 24 mAh lithium cobalt oxide/graphite coin cell was selected for cyclic EIS testing. The EIS measurements were carried out over a frequency range from 0.01 Hz to 100 kHz, with a voltage perturbation amplitude of 5 mV. Measurements were performed at every 10% increment of SOC, and the corresponding voltage and current profiles are depicted in Figure 3.
Figure 3. Voltage and current curves during in-situ EIS testing
3. Data Analysis
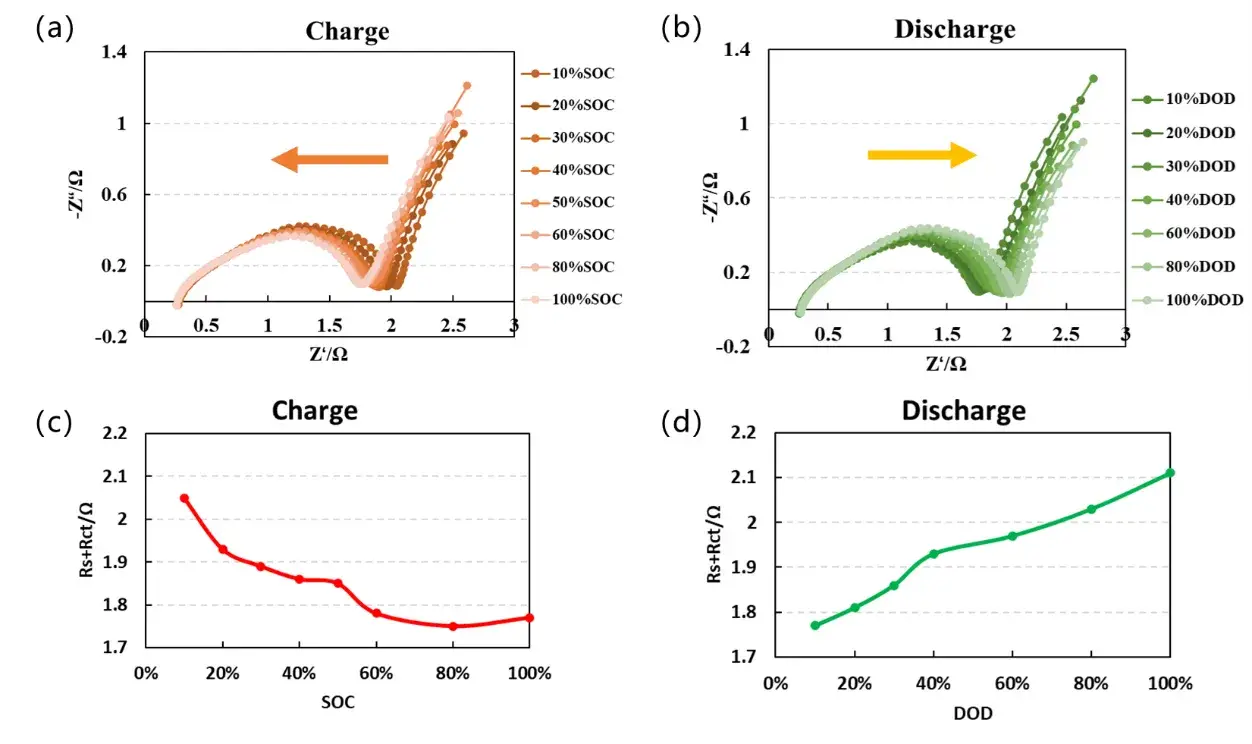
EIS measurements were performed on the coin cell at various SOCs during both the charging and discharging processes, with the results shown in Figure 4. Analysis of the Nyquist plots in panels (a) and (b) indicates that as the charging SOC increases, the semicircular portion of the impedance spectrum gradually shifts leftward, whereas with increasing depth of discharge (DOD), the semicircle shifts rightward. By extracting the real component of the impedance at approximately 1 Hz, the curves in panels (c) and (d) reveal that impedance decreases progressively during charging and increases during discharging. This behavior is consistent with the “bathtub curve” characteristic of a battery cell’s internal resistance, primarily attributed to the reduction in charge transfer resistance at high SOC as lithium ions are deintercalated from the electrode materials. The in-situ EIS testing method facilitates the rapid acquisition of impedance variation trends across different cell components under varying operating conditions, thereby assisting researchers in the comprehensive analysis of material performance.
Figure 4. EIS curves at different SOCs during charging and discharging processes
4. Conclusion
This study employed IEST electrochemical analysis system ERT to perform in-situ EIS measurements on coin cells during charge–discharge cycles at various SOCs. The results demonstrated that the impedance decreases with increasing charging SOC and increases with increasing DOD. The application of this in-situ EIS testing methodology can significantly aid researchers in the detailed analysis of both material properties and battery cell performance.
5. References
[1] Pouyan Shafiei Sabet, Dirk Uwe Sauer. Separation of predominant processes in electrochemical impedance spectra of lithium-ion batteries with nickel manganese cobalt cathodes. Journal of Power Sources, 425 (2019) 121–129.
6. Electrochemical Instruments Provider Recommend:IEST Instrument

Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.