IEST Instruments
The Galvanostatic Intermittent Titration Technique(GITT), a transient electrochemical measurement method, enables quantitative analysis of lithium-ion diffusion dynamics in electrode materials by correlating potential transients with time [1] [2] .
Lithium-ion batteries (LIBs) are rocking-chair-type secondary batteries that operate via the reversible migration of lithium ions between the cathode and anode (Figure 1). During charging, lithium ions deintercalate from the cathode, traverse the electrolyte, and intercalate into the anode. Concurrently, electrons flow from the cathode to the anode through an external circuit, generating a current. During discharging, the reverse occurs: lithium ions deintercalate from the anode, return to the cathode via the electrolyte, and electrons flow back through the external circuit to power external devices. The diffusion rate and efficiency of lithium ions critically influence key battery performance metrics, including charge/discharge rates, cycle life, and performance under extreme temperatures.
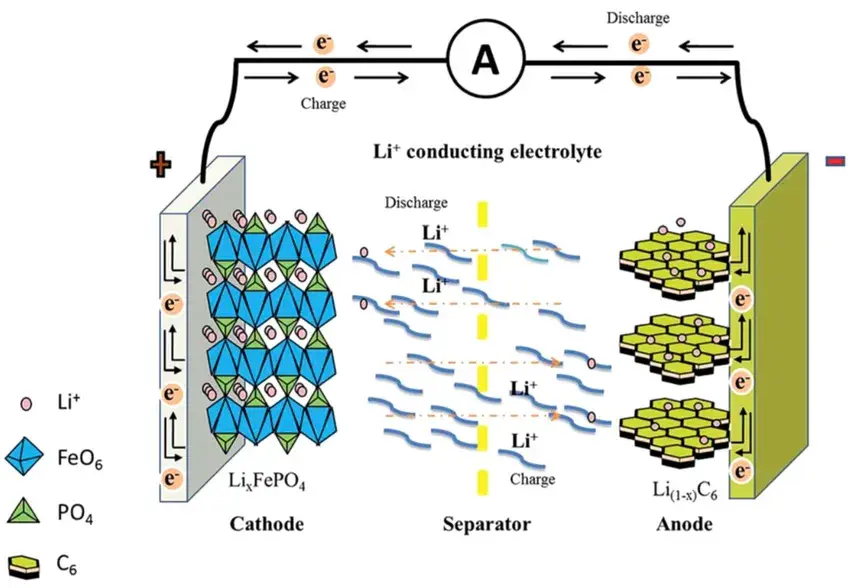
Figure 1. Schematic illustration of lithium-ion battery operation.
The GITT testing procedure consists of a series of “pulse–constant current–relaxation” cycles (see Figure 2). In each cycle, a constant current is applied to charge or discharge the battery for a predetermined duration, followed by a relaxation period during which the current is interrupted and the voltage is continuously recorded. The key to this method is maintaining a constant current and achieving precise voltage measurements. During the relaxation phase, sufficient time is allowed for lithium ions to thoroughly diffuse within the active material, enabling the calculation of the diffusion coefficient based on the voltage-time relationship. To adhere to the GITT assumption that “the diffusion process predominantly occurs in the surface layer of the solid material,” the testing conditions must meet the following criteria:
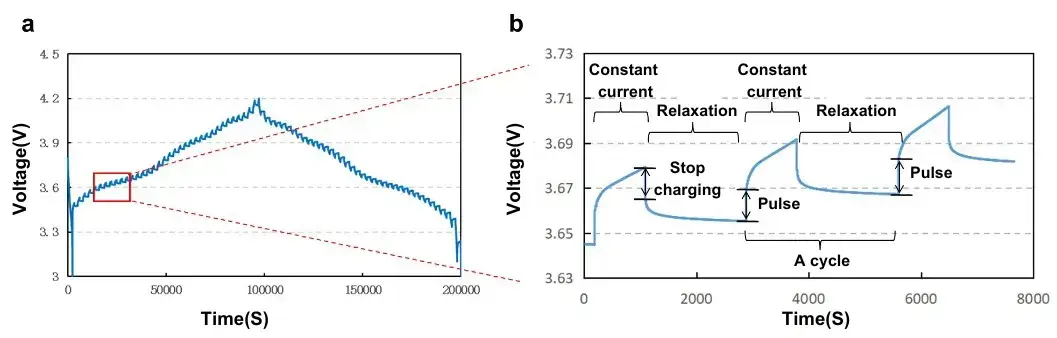
Figure 2. (a) GITT test profile and (b) magnified view of a single polarization-relaxation cycle.
The lithium-ion diffusion coefficient (D) is derived from GITT data using the following equation:
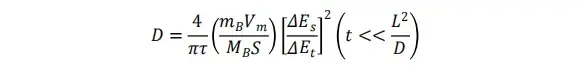
where:
By substituting the appropriate physical parameters and the measured ΔEs and ΔEt values from each “pulse–relaxation” cycle into the equation, the lithium ion diffusion coefficient can be determined. In practical measurements, the observed voltage change comprises not only the contribution from surface diffusion but also the voltage variation due to changes in the state of charge (SOC). Although reducing the pulse duration can theoretically improve the accuracy of GITT, shorter pulses result in very small ΔEs values, necessitating high-precision instrumentation to minimize noise. IEST’s self-developed potentiostat integrates an 8-channel high-precision measurement system (0.01% accuracy) to ensure reliable GITT data acquisition.
Using GITT, researchers investigated the variation of the lithium ion diffusion coefficient in LiNi₀.₈Co₀.₁Mn₀.₁O₂ (NCM811) during charge–discharge cycles [3]. The results revealed significant changes in the lithium ion diffusion coefficient (D<sub>Li⁺</sub>) under varying SOC conditions. During charging, D<sub>Li⁺</sub> ranged from 10⁻⁸ to 10⁻⁹ cm²/s, whereas during discharging, it varied between 10⁻⁷ and 10⁻¹¹ cm²/s. In the initial stage of charging, D<sub>Li⁺</sub> increased with the extraction of lithium ions, reaching a maximum when the lithium content was approximately 0.5, and then gradually decreased. When the lithium content fell below 0.2, the diffusion coefficient dropped sharply. In contrast, during discharging, D<sub>Li⁺</sub> was extremely high at the beginning, then decreased slightly and remained relatively high with continued lithium intercalation. Notably, when the lithium ion intercalation reached 0.8, D<sub>Li⁺</sub> decreased abruptly by three orders of magnitude, which explains the capacity loss observed in the first cycle.

Figure 3. (a) GITT curves and (b) DLi+DLi+ evolution for NCM811 during the first cycle.
High-entropy doping (Cr, Mn, Fe, Zn, Al) in Na₃V₂(PO₄)₃ (NVP) produced Na₃V₁.₈(CrMnFeZnAl)₀.₂(PO₄)₃ (HE-NVP-0.2), which exhibited improved Na⁺ diffusion kinetics [4]. GITT analysis confirmed enhanced DNa+DNa+ in HE-NVP-0.2 (Figures 4a, 4b), correlating with superior rate performance in half-cells (Figure 4c).

Figure 4. GITT profiles and DNa+DNa+ for (a) NVP and (b) HE-NVP-0.2; (c) Rate performance comparison.
Lithium-ion diffusion kinetics in electrode materials govern macroscopic battery performance. Segmenting electrochemical reactions by state-of-charge (SOC) enables identification of polarization-limiting factors at each stage. GITT provides a robust framework for quantifying DD, making it indispensable for battery R&D.
Based on the importance of GITT to the study of lithium-ion batteries, IEST has independently developed a high-precision electrochemical performance analyzer, which integrates the GITT test into the conventional charging and discharging equipment, templates the work steps, makes the setup simple, and makes the operation easy to improve the testing efficiency. The device also integrates CV (Cyclic Voltammetry), EIS (Electrochemical impedance spectroscopy) and LSV (linear scanning voltammetry) modules to enable R&D personnel to quickly conduct relevant electrochemical performance studies. In addition, the IEST electrochemical analyzer is equipped with advanced data processing and analysis software that enables real-time processing and multi-dimensional analysis of complex electrochemical data.
Figure 5. IEST electrochemical analyzer
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.
Please fill out the form below and we will contact you asap!

