-
iestinstrument
Summary of Electrochemical Impedance Spectroscopy(EIS) for Lithium-Ion Batteries
1. Introduction
Electrochemical measurement techniques, based on quantifying electrical parameters such as potential, conductivity, and current in electrochemical systems, facilitate both qualitative and quantitative analyses of system components by investigating the relationships between the measured signals and the system itself. Common methods include constant current/constant potential techniques, chronoamperometry/chronopotentiometry, voltammetry, and electrochemical impedance spectroscopy (EIS) [1].
Compared with other electrochemical methods, EIS induces minimal disturbance to the intrinsic state of the system while its wide frequency range reveals detailed information about the internal electrochemical processes. Consequently, EIS has been extensively applied in the field of lithium-ion batteries. Numerous researchers have leveraged precise EIS measurements and analyses to explore various aspects of lithium-ion batteries. This article analyzes EIS using the example of lithium-ion batteries.
2. Fundamentals of Electrochemical Impedance Spectroscopy
Electrochemical impedance spectroscopy (EIS), also known as electrochemical AC impedance spectroscopy, is a critical technique for assessing the electrochemical performance of lithium-ion batteries. In EIS, a small-amplitude sinusoidal voltage signal is applied across the system as a perturbation, and the resulting sinusoidal current response is measured. By comparing the input (voltage) and output (current) signals, the frequency-dependent impedance Z(ω)=X/YZ(\omega) = X/YZ(ω)=X/Y is determined. Here, a lithium-ion battery—being a system that satisfies linearity, stability, and causality conditions—is excited using a series of sinusoidal voltage signals (typically 5 mV amplitude over a frequency range of 0.1 Hz to 100 Hz), resulting in a complete impedance spectrum.
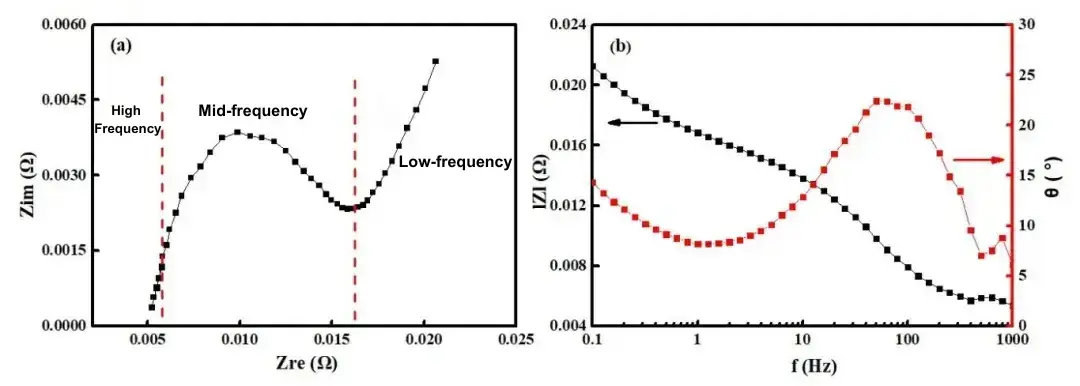
The data are typically represented using Bode and Nyquist plots. The Nyquist plot displays the real part of the impedance (ZRe) on the horizontal axis and the negative imaginary part (−ZIm) on the vertical axis, thereby visually delineating the time constants of various reaction processes within the system. The Bode plot, on the other hand, shows how phase angle and magnitude vary with frequency and is often employed to assess the performance and stability of electronic circuits. Figure 1 illustrates the Nyquist and Bode plots for a 2500 mAh lithium iron phosphate battery over a frequency range of 0.1 Hz to 1 kHz (with frequency decreasing from left to right). At 1 kHz, the imaginary component is nearly zero; as frequency decreases, the real component increases gradually, while the negative imaginary part initially increases, then decreases, and finally increases again. The EIS curve is composed of three segments: two irregular semicircular arcs in the high- and mid-frequency regions and an inclined line in the low-frequency region. Analysis reveals that the high-frequency semicircle corresponds to the migration and diffusion of lithium ions through multiple layers and the SEI film; the mid-frequency semicircle represents the charge transfer process; and the low-frequency inclined line reflects the solid-state diffusion of lithium ions within the active electrode material[2].
Figure 1. (a) Nyquist plot and (b) Bode plot of a LiFePO₄ battery.
3. Analysis of Electrochemical Impedance Spectroscopy
3.1 Components of the EIS Spectrum
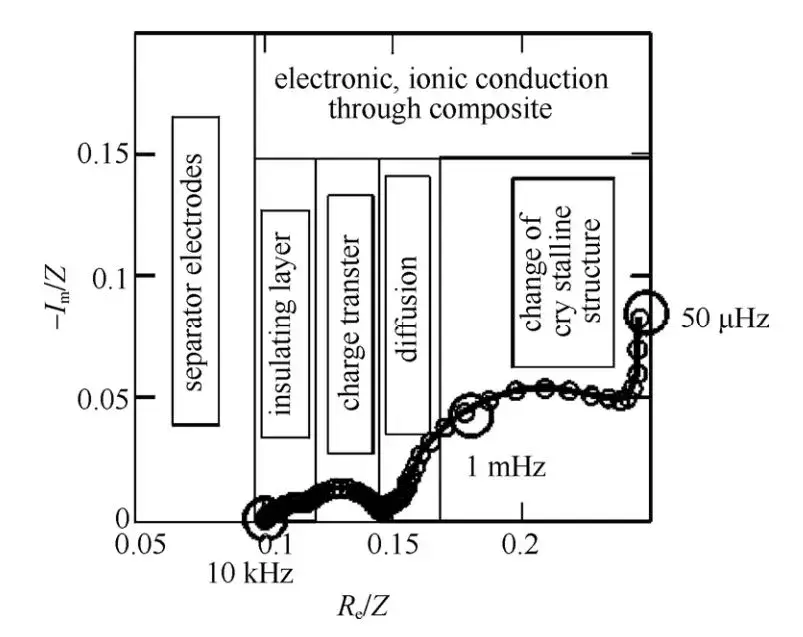
A typical EIS spectrum for a lithium-ion battery can be divided into five regions (see Figure 2):
- Ultra-high frequency region (>10 kHz):
This region reflects the ohmic resistance associated with the transport of Li+ ions and electrons through the electrolyte, porous separator, wiring, and active material particles. In the EIS spectrum, it appears as a single point and is represented by the resistor RsR_sRs. - High-frequency region:
Characterized by a semicircular arc, this region is related to the diffusion and migration of Li+ ions through the insulating layer on the surface of active material particles. It can be modeled by a parallel Rsei/Csei circuit, where Rsei denotes the resistance associated with Li+ migration through the SEI layer. - Mid-frequency region:
This semicircle is associated with the charge transfer process and is represented by an Rct/Cdl parallel circuit. Rct denotes the charge transfer resistance (also known as electrochemical reaction resistance), while Cdl represents the double-layer capacitance. - Low-frequency region:
Represented by an inclined line, this region pertains to the solid-state diffusion of Li+ ions within the active material particles and is modeled by the Warburg impedance Zw. - Very low-frequency region (<0.01 Hz):
This region consists of a semicircle associated with changes in the crystal structure of active material particles or the formation of new phases, coupled with a vertical line related to the accumulation and consumption of Li+ ions. It can be modeled by a series combination of a parallel Rb/Cb circuit (representing structural changes in the active material) and an insertion capacitance Cint (representing Li+ accumulation/consumption).
Figure 2. Typical Electrochemical Impedance Spectroscopy of Li+ Intercalation and Deintercalation Processes in Compound Electrodes[3]
3.2 Equivalent Circuit Models
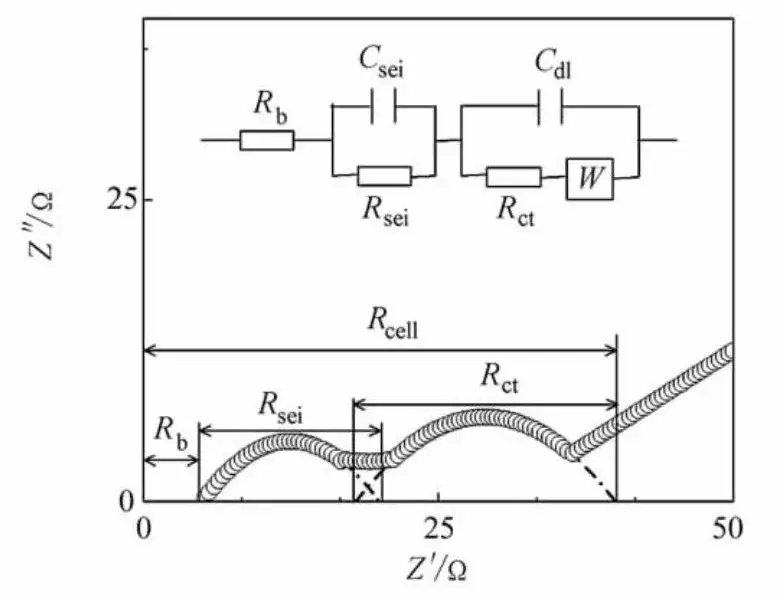
A lithium-ion battery can be modeled as an electrical circuit comprising resistors, inductors, and capacitors. The equivalent circuit model simplifies the battery to simulate the dynamic processes occurring within the electrochemical system. Figure 3 shows a commonly used equivalent circuit model for lithium-ion batteries. In this model, Rs represents the ohmic resistance; Rsei and Csei represent the resistance and capacitance of the SEI layer (corresponding to the high-frequency semicircle); Rct and Cdl denote the charge transfer resistance and double-layer capacitance (corresponding to the mid-frequency semicircle); and WWW represents the Warburg impedance, which models the diffusion resistance of Li+ ions within the electrode material and appears as a 45° line relative to the real axis in the complex plane. Software packages such as Zview, ZSimpWin, EIS300, LEVMW, Impedance Spectroscopy, and Autolab Nova are typically used to select the appropriate equivalent circuit model and fit the EIS data to extract the impedance values corresponding to each process stage.
Figure 3. Lithium-ion battery impedance spectrum and equivalent circuit model [4]
4. Applications
Electrochemical impedance spectroscopy is an indispensable tool for investigating interfacial processes in electrochemical systems and has wide-ranging applications in lithium-ion battery research. Its applications include, but are not limited to:
- Characterization of Electrode Materials:
Testing the EIS of electrode materials in various electrolytes enables evaluation of their conductivity and reactivity, thereby assisting in the optimization of electrode design and fabrication. - Investigation of Internal Battery Processes:
EIS provides insights into internal processes such as ionic migration within the electrolyte, charge transfer between the electrode and electrolyte, and the kinetics of electrochemical reactions, all of which are crucial for optimizing battery performance and longevity. - Battery State and Fault Diagnosis:
By monitoring changes in the internal electrochemical processes (e.g., loss of active material, variations in electrolyte concentration, or temperature fluctuations), EIS can help diagnose battery state and identify faults. - Evaluation of Cycle Life:
Measuring impedance changes during cycling allows for assessment of the battery’s cycle life and degradation mechanisms, facilitating the development of optimal usage and maintenance strategies. - Study of Thermal Runaway Characteristics:
EIS can be utilized to examine how temperature, current density, and capacity affect battery impedance, thus contributing to the evaluation of battery safety and the design of safer battery systems. - Investigation of Electrolyte Properties:
By measuring conductivity and ion migration characteristics, EIS enables detailed studies of electrolyte properties such as ion concentration, mobility, and diffusion coefficients.
In summary, the judicious use of EIS enhances our understanding of battery systems, thereby advancing battery research and development, improving performance, and supporting the effective management and application of battery systems.
5. Summary
Building on the extensive application of EIS in lithium-ion batteries, IEST has independently developed an Electrochemical Performance Analyzer (Figure 4). In addition to conventional charge–discharge functionality, this instrument integrates modules for cyclic voltammetry (CV) and EIS, enabling EIS measurements during battery cycling (as shown in Figure 5). As illustrated in Figure 4(b), the EIS module—operating as an independent testing unit—can be positioned after any process step. This flexibility allows for EIS testing after every N cycles or at specific states-of-charge (SOC) during charging/discharging, eliminating the need for battery disassembly or relocation and thereby enhancing both testing efficiency and accuracy.
Figure 4. IEST Electrochemical Performance Analyzer (a) and Cyclic EIS Testing Steps (b)
Figure 5. EIS Data During Cell Cycling
6. References
[1] Ning B,Cao B,Wang B,et al. Adaptive Sliding Mode Observers for Lithium-ion Battery State Estimation Based on Parameters Identified Online[J].
[2] Zhang Jinlong, Tong Wei, Qi Hanhong, et al. Application of Square Root Sigma Point Kalman Filter to SOC Estimation of Li FePO_4 Battery Pack[J].Proceedings of the CSEE,2016,36(22):6246-6253.
[3] Barsoukov E , Macdonald R J .Impedance Spectroscopy: Theory, Experiment, and Applications[M].Wiley-Interscience, 2005.
[4] Zhang S S, Xu K, Jow T R. Electrochemical impedance study on the low temperature of Li-ion batteries[J]. Electro-chim Acta, 2004, 49 ( 7) : 1057-1061.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.